POMALYST® (pomalidomide) is used in several
FDA-approved therapy combinations to treat
relapsed/refractory multiple myeloma
Understanding Combinations
Your doctor may choose to prescribe you a doublet or triplet drug regimen containing POMALYST. Doublets are a combination of 2 medications, and triplets are a combination of 3 medications.
Regardless of the combination, a POMALYST-containing regimen works with your immune system to help you fight your relapsed/refractory multiple myeloma.
POMALYST Combination Options
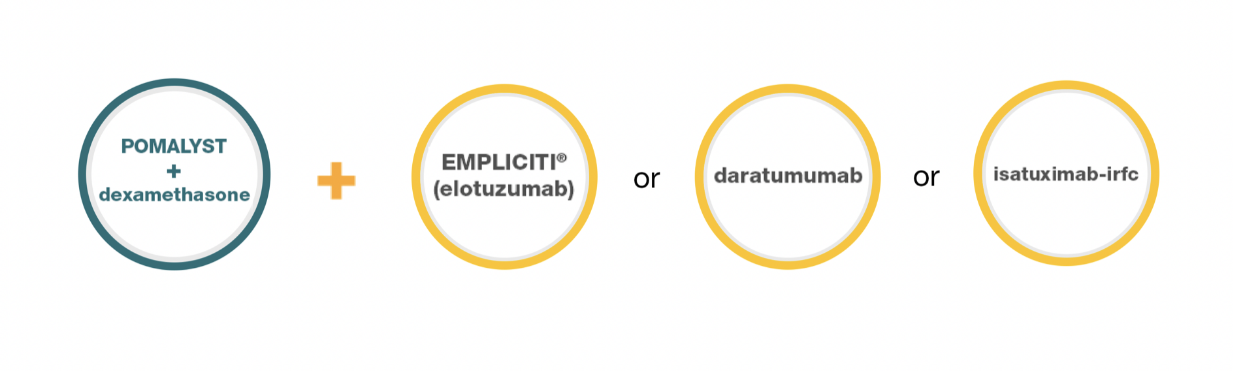

It’s important to talk to your doctor about these combinations and the associated risks and benefits. POMALYST may not work for everyone so be sure to ask your doctor if a POMALYST-containing treatment option is right for you.
POMALYST + dexamethasone + EMPLICITI® (elotuzumab)
Please talk to your doctor and/or read full Prescribing Information, including Boxed WARNINGS and Medication Guide for a complete discussion of Important Safety Information.
What is POMALYST® (pomalidomide)?
POMALYST is a prescription medicine, taken with the medicine dexamethasone, used to treat adults with multiple myeloma who have previously received at least 2 medicines to treat multiple myeloma, including a proteasome inhibitor and lenalidomide, and whose disease has become worse during treatment or within 60 days of finishing the last treatment.
What is EMPLICITI® (elotuzumab)?
EMPLICITI is a prescription medicine used to treat multiple myeloma in combination with the medicines:
- POMALYST® (pomalidomide) and dexamethasone in adults who have received at least two prior treatments including REVLIMID and a proteasome inhibitor.
It is not known if POMALYST or EMPLICITI is safe and effective in children.
What are the possible side effects of POMALYST and EMPLICITI?
- See “What is the most important information I should know about POMALYST?”
- POMALYST and EMPLICITI can cause serious side effects, including:
Low white blood cells (neutropenia), low platelets (thrombocytopenia), and low red blood cells (anemia) are common with POMALYST, but can also be serious. You may need a blood transfusion or certain medicines if your blood counts drop too low. Your blood counts should be checked by your healthcare provider (HCP) weekly for the first 8 weeks of treatment and monthly after that.
Severe liver problems, including liver failure and death. Your HCP should do blood tests to check your liver function during your treatment with POMALYST and EMPLICITI. Tell your HCP right away if you develop any of the following symptoms of liver problems: yellowing of your skin or the white parts of your eyes (jaundice); dark or brown (tea-colored) urine; color changes in your stool; pain or swelling on the upper right side of your stomach area (abdomen); confusion; bleeding or bruising more easily than normal or feeling very tired.
Infusion Reactions. Infusion reactions can happen during your infusion or within 24 hours after your infusion of EMPLICITI. Your healthcare provider will give you medicines before each infusion of EMPLICITI to help reduce the risk of an infusion reaction. If you have an infusion reaction while receiving EMPLICITI, your healthcare provider will slow or stop your infusion and treat your reaction. If you have a severe infusion reaction your healthcare provider may stop your treatment completely. Tell your healthcare provider or get medical help right away if you have any of these symptoms after your infusion with EMPLICITI: fever, chills, rash, chest pain, trouble breathing, dizziness, or light-headedness.
Infections. Those receiving EMPLICITI with POMALYST and dexamethasone may develop infections; some can be serious. Tell your healthcare provider right away if you have any of the signs and symptoms of an infection, including fever, flu-like symptoms, cough, shortness of breath, burning with urination, or a painful skin rash.
Severe allergic and severe skin reactions can happen with POMALYST and may cause death.
Dizziness and confusion. Avoid taking other medicines that may cause dizziness and confusion during treatment with POMALYST. Avoid situations that require you to be alert until you know how POMALYST affects you.
Nerve damage. Stop taking POMALYST and call your HCP if you develop numbness, tingling, pain, or a burning sensation in your hands, legs, or feet.
Risk of new cancers (malignancies). New cancers, including certain blood cancers (acute myelogenous leukemia or AML) have been seen in people who received POMALYST. Those receiving EMPLICITI with POMALYST and dexamethasone have a risk of developing new cancers. Your healthcare provider will check you for new cancers during your treatment with POMALYST, EMPLICITI and dexamethasone. Talk with your HCP about your risk of developing new cancers.
Tumor Lysis Syndrome (TLS). TLS is caused by the fast breakdown of cancer cells. TLS can cause kidney failure and the need for dialysis treatment, abnormal heart rhythm, seizure, and sometimes death. Your HCP may do blood tests to check you for TLS. - The most common side effects of POMALYST include tiredness and weakness, constipation, nausea, diarrhea, shortness of breath, upper respiratory tract infection, back pain, and fever.
- The most common side effects of EMPLICITI when used with POMALYST and dexamethasone include constipation and high blood sugar.
- These are not all the possible side effects of POMALYST and EMPLICITI. Your HCP may tell you to decrease your dose, temporarily stop or permanently stop taking POMALYST if you develop certain serious side effects during treatment. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Please read the Patient Information and the full Prescribing Information for EMPLICITI, and Medication Guide.
Important Safety Information for POMALYST and EMPLICITI
WARNINGS: Risk to unborn babies, and blood clots.
What is the most important information I should know about POMALYST?
Before you begin taking POMALYST, you must read and agree to all of the instructions in the POMALYST REMS® program. Further information about the POMALYST REMS program is available at www.pomalystrems.com or by telephone at
POMALYST can cause serious side effects, including:
- Possible birth defects (deformed babies) or death of an unborn baby. Females who are pregnant or plan to become pregnant must not take POMALYST.
- POMALYST is similar to the medicine thalidomide (THALOMID®), which is known to cause severe life-threatening birth defects. POMALYST has not been tested in pregnant females. POMALYST has harmed unborn animals in animal testing.
- Females must not get pregnant:
- For at least 4 weeks before starting POMALYST
- While taking POMALYST
- During any breaks (interruptions) in your treatment with POMALYST
- For at least 4 weeks after stopping POMALYST
- Females who can become pregnant:
- Will have pregnancy tests weekly for 4 weeks, then every 4 weeks if your menstrual cycle is regular, or every 2 weeks if your menstrual cycle is irregular. If you miss your period or have unusual bleeding, you will need to have a pregnancy test and receive counseling.
- Must agree to use 2 acceptable forms of effective birth control at the same time, for at least 4 weeks before, while taking, during any breaks (interruptions) in treatment, and for at least 4 weeks after stopping POMALYST.
- Talk with your healthcare provider to find out about options for acceptable forms of birth control that you may use to prevent pregnancy during and after treatment with POMALYST.
- If you become pregnant while taking POMALYST, stop taking it right away and call your healthcare provider. If your healthcare provider is not available, you can call the REMS Call Center at 1-888-423-5436. Healthcare providers and patients should report all cases of pregnancy to:
- FDA MedWatch at 1-800-FDA-1088
- REMS Call Center at 1-888-423-5436
There is a pregnancy exposure registry that monitors the outcomes of females who take POMALYST during pregnancy, or if their male partner takes POMALYST and they are exposed during pregnancy. You can enroll in this registry by calling the REMS Call Center at the phone number listed above.
- POMALYST can pass into human semen:
- Males, including those who have had a vasectomy, must always use a latex or synthetic condom during any sexual contact with a pregnant female or a female that can become pregnant while taking POMALYST, during any breaks (interruptions) in your treatment with POMALYST, and for 4 weeks after stopping POMALYST.
- Do not have unprotected sexual contact with a female who is or could become pregnant. Tell your healthcare provider if you do have unprotected sexual contact with a female who is or could become pregnant.
- Do not donate sperm while taking POMALYST, during any breaks (interruptions) in your treatment, and for 4 weeks after stopping POMALYST. If a female becomes pregnant with your sperm, the baby may be exposed to POMALYST and may be born with birth defects.
Men, if your female partner becomes pregnant, you should call your healthcare provider right away.
- Do not donate blood while you take POMALYST, during any breaks (interruptions) in your treatment, and for 4 weeks after stopping POMALYST. If someone who is pregnant gets your donated blood, her baby may be exposed to POMALYST and may be born with birth defects.
- Blood clots in your arteries, veins, and lungs, heart attack, and stroke can happen if you take POMALYST.
- Most people who take POMALYST will also take a blood thinner medicine to help prevent blood clots.
- Before taking POMALYST, tell your healthcare provider:
- If you have had a blood clot in the past.
- If you have high blood pressure, smoke, or if you have been told you have a high level of fat in your blood (hyperlipidemia).
- About all the medicines you take. Certain other medicines can also increase your risk for blood clots.
Call your healthcare provider or get medical help right away if you get any of the following during treatment with POMALYST:
- Signs or symptoms of a blood clot in the lung, arm, or leg may include: shortness of breath, chest pain, or arm or leg swelling.
- Signs or symptoms of a heart attack may include: chest pain that may spread to the arms, neck, jaw, back, or stomach area (abdomen); feeling sweaty, shortness of breath, feeling sick, or vomiting.
- Signs or symptoms of stroke may include: sudden numbness or weakness, especially on one side of the body, severe headache or confusion, or problems with vision, speech, or balance.
- A red, itchy skin rash
- Peeling of your skin or blisters
- Severe itching
- Fever
Get emergency medical help right away if you develop any of the following signs or symptoms during treatment with POMALYST:
- swelling of your lips, mouth, tongue, or throat
- trouble breathing or swallowing
- raised red areas on your skin (hives)
- a very fast heartbeat
- you feel dizzy or faint
Who should not take POMALYST?
Do not take POMALYST if you:
- Are pregnant, plan to become pregnant, or become pregnant during treatment with POMALYST. See “What is the most important information I should know about POMALYST?”
- Are allergic to pomalidomide or any of the ingredients in POMALYST.
What should I tell my healthcare provider (HCP) before taking EMPLICITI or POMALYST?
Before you take EMPLICITI or POMALYST, tell your healthcare provider about all of your medical conditions, including if you:
- smoke cigarettes (POMALYST may not work as well in people who smoke)
- have liver problems
- have kidney problems or receive hemodialysis treatment
- have thyroid problems
- have an infection
- are pregnant or plan to become pregnant. It is not known if EMPLICITI may harm your unborn baby. However, POMALYST may cause birth defects or death of an unborn baby.
- are breastfeeding. Do not breastfeed during treatment with EMPLICITI and POMALYST and dexamethasone.
- Tell your HCP about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. POMALYST and other medicines may affect each other, causing serious side effects. Talk with your HCP before taking any new medicines.
- Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist.
How should I take POMALYST?
Take POMALYST exactly as prescribed and follow all the instructions of the POMALYST REMS program.
- Swallow POMALYST capsules whole with water 1 time a day. Do not break, chew, or open capsules.
- POMALYST may be taken with or without food.
- Take POMALYST at the same time each day.
- If you are on hemodialysis, take POMALYST after hemodialysis on hemodialysis days.
- Do not open POMALYST capsules or handle them any more than needed. If you touch a broken POMALYST capsule or the medicine in the capsule, wash the area of your body right away with soap and water.
- If you miss a dose of POMALYST and it has been less than 12 hours since your regular time, take POMALYST as soon as you remember. If it has been more than 12 hours, just skip your missed dose. Do not take 2 doses at the same time.
- If you take too much POMALYST, call your HCP right away.
- Do not share POMALYST with other people. It may cause birth defects and other serious problems.
What are the possible side effects of POMALYST and EMPLICITI?
- See “What is the most important information I should know about POMALYST?”
- POMALYST and EMPLICITI can cause serious side effects, including:
- Low white blood cells (neutropenia), low platelets (thrombocytopenia), and low red blood cells (anemia) are common with POMALYST, but can also be serious. You may need a blood transfusion or certain medicines if your blood counts drop too low. Your blood counts should be checked by your healthcare provider (HCP) weekly for the first 8 weeks of treatment and monthly after that.
- Severe liver problems, including liver failure and death. Your HCP should do blood tests to check your liver function during your treatment with EMPLICITI and POMALYST. Tell your HCP right away if you develop any of the following symptoms of liver problems: yellowing of your skin or the white parts of your eyes (jaundice); dark or brown (tea-colored) urine; color changes in your stool; pain or swelling on the upper right side of your stomach area (abdomen); confusion; bleeding or bruising more easily than normal, or feeling very tired.
- Infusion Reactions. Infusion reactions can happen during your infusion or within 24 hours after your infusion of EMPLICITI. Your healthcare provider will give you medicines before each infusion of EMPLICITI to help reduce the risk of an infusion reaction. If you have an infusion reaction while receiving EMPLICITI, your healthcare provider will slow or stop your infusion and treat your reaction. If you have a severe infusion reaction your healthcare provider may stop your treatment completely. Tell your healthcare provider or get medical help right away if you have any of these symptoms after your infusion with EMPLICITI: fever, chills, rash, chest pain, trouble breathing, dizziness, or light-headedness.
- Infections. Those receiving EMPLICITI with POMALYST and dexamethasone may develop infections; some can be serious. Tell your healthcare provider right away if you have any of the signs and symptoms of an infection, including: fever, flu-like symptoms, cough, shortness of breath, burning with urination, or a painful skin rash. Severe allergic and severe skin reactions can happen with POMALYST and may cause death.
- Dizziness and confusion. Avoid taking other medicines that may cause dizziness and confusion during treatment with POMALYST. Avoid situations that require you to be alert until you know how POMALYST affects you.
- Nerve damage. Stop taking POMALYST and call your HCP if you develop numbness, tingling, pain, or a burning sensation in your hands, legs, or feet.
- Risk of new cancers (malignancies). New cancers, including certain blood cancers (acute myelogenous leukemia or AML) have been seen in people who received POMALYST. Those receiving EMPLICITI with POMALYST and dexamethasone have a risk of developing new cancers. Your healthcare provider will check you for new cancers during your treatment with POMALYST, EMPLICITI and dexamethasone. Talk with your HCP about your risk of developing new cancers.
- Tumor Lysis Syndrome (TLS). TLS is caused by the fast breakdown of cancer cells. TLS can cause kidney failure and the need for dialysis treatment, abnormal heart rhythm, seizure, and sometimes death. Your HCP may do blood tests to check you for TLS.
- The most common side effects of POMALYST include tiredness and weakness, constipation, nausea, diarrhea, shortness of breath, upper respiratory tract infection, back pain, and fever.
- The most common side effects of EMPLICITI when used with POMALYST and dexamethasone include constipation and high blood sugar.
- These are not all the possible side effects of EMPLICITI and POMALYST. Your HCP may tell you to decrease your dose, temporarily stop or permanently stop taking POMALYST if you develop certain serious side effects during treatment. Call your doctor for medical advice about side effects. You may report side effects to FDA at
1-800-FDA-1088.
Please read the Patient Information in the full Prescribing Information for EMPLICITI, and the Medication Guide in the full Prescribing Information for POMALYST, including Boxed WARNINGS for POMALYST.
Important Safety Information
POMALYST + dexamethasone + daratumumab
Information about POMALYST + dexamethasone + daratumumab does not appear in the POMALYST full Prescribing Information. Please talk to your doctor and/or see the daratumumab full Prescribing Information including Patient Information for a complete discussion of Important Safety Information at www.darzalex.com.
Indication and Usage
Daratumumab is a prescription medicine used, in combination with POMALYST and dexamethasone, to treat multiple myeloma in people who have received at least two prior medicines, including lenalidomide and a proteasome inhibitor.
It is not known if daratumumab is safe and effective in children.
Most common adverse reactions (20% or higher)
The most frequent adverse reactions with POMALYST + dexamethasone + daratumumab were low white blood cell count, low platelet count, low red blood cell count, infusion-related reactions, fatigue, upper respiratory tract infection, cough, diarrhea, constipation, shortness of breath, nausea, muscle spasms, fever, back pain, insomnia, joint pain, vomiting, dizziness, and chills.
The most common treatment-related changes to blood levels with POMALYST + dexamethasone + daratumumab were low red blood cells, low platelets, low neutrophils, and low lymphocytes.
Serious side effects of daratumumab
Daratumumab may cause serious side effects, including infusion-related reactions, changes in blood tests, and decreases in blood cell counts.
These are not all of the possible side effects of daratumumab. If you have questions, talk to your healthcare provider to learn more.
Important Safety Information for daratumumab
What is daratumumab?
Daratumumab is a prescription medicine used to treat adults with multiple myeloma:
- in combination with the medicines pomalidomide and dexamethasone in people who have received at least two prior medicines to treat multiple myeloma, including lenalidomide and a proteasome inhibitor.
- alone in people who have received at least three prior medicines, including a proteasome inhibitor and an immunomodulatory agent, or did not respond to a proteasome inhibitor and an immunomodulatory agent.
It is not known if daratumumab is safe and effective in children.
Do not receive daratumumab:
- if you have a history of a severe allergic reaction to daratumumab or any of the ingredients in daratumumab. See the end of this page for a complete list of ingredients in daratumumab.
Before you receive daratumumab, tell your healthcare provider about all of your medical conditions, including if you:
- have a history of breathing problems
- have had shingles (herpes zoster)
- have ever had or might now have a hepatitis B infection as daratumumab could cause hepatitis B virus to become active again. Your healthcare provider will check you for signs of this infection before, during and for some time after treatment with daratumumab. Tell your healthcare provider right away if you get worsening tiredness or yellowing of your skin or white part of your eyes.
- are pregnant or plan to become pregnant. daratumumab may harm your unborn baby. Tell your healthcare provider right away if you become pregnant or think that you may be pregnant during treatment with daratumumab.
- Females who are able to become pregnant should use an effective method of birth control during treatment and for at least 3 months after your final dose of daratumumab. Talk to your healthcare provider about birth control methods that you can use during this time
- Before starting daratumumab in combination with lenalidomide, pomalidomide, or thalidomide, females and males must agree to the instructions in the lenalidomide, pomalidomide, or thalidomide REMS program.
- The lenalidomide, pomalidomide, and thalidomide REMS has more information about effective methods of birth control, pregnancy testing, and blood donation for females who can become pregnant.
- For males who have female partners who can become pregnant, there is information in the lenalidomide, pomalidomide, and thalidomide REMS about sperm donation and how lenalidomide, pomalidomide, and thalidomide can pass into human semen.
- are breastfeeding or plan to breastfeed. It is not known if daratumumab passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How will I receive daratumumab?
- Daratumumab may be given alone or together with other medicines used to treat multiple myeloma.
- Daratumumab will be given to you by your healthcare provider by intravenous (IV) infusion into your vein.
- Your healthcare provider will decide the time between doses as well as how many treatments you will receive.
- Your healthcare provider will give you medicines before each dose of daratumumab and after each dose of daratumumab to help reduce the risk of infusion-related reactions.
- If you miss any appointments, call your healthcare provider as soon as possible to reschedule your appointment.
What are the possible side effects of daratumumab?
Daratumumab may cause serious reactions, including:
- Infusion-related reactions. Infusion-related reactions are common with daratumumab and can be severe or serious. Your healthcare provider may temporarily stop your infusion or completely stop treatment with daratumumab if you have infusion-related reactions. Get medical help right away if you get any of the following symptoms:
- shortness of breath or trouble breathing
- dizziness or lightheadedness (hypotension)
- cough
- wheezing
- throat tightness
- runny or stuffy nose
- headache
- itching
- nausea
- vomiting
- chills
- fever
Changes in blood tests. Daratumumab can affect the results of blood tests to match your blood type. These changes can last for up to 6 months after your final dose of daratumumab. Your healthcare provider will do blood tests to match your blood type before you start treatment with daratumumab. Tell all of your healthcare providers that you are being treated with daratumumab before receiving blood transfusions.
Decreases in blood cell counts. Daratumumab can decrease white blood cell counts, which help fight infections, and blood cells called platelets, which help to clot blood. Your healthcare provider will check your blood cell counts during treatment with daratumumab. Tell your healthcare provider if you develop fever or have signs of bruising or bleeding.
The most common side effects of daratumumab include:
- tiredness
- nausea
- diarrhea
- shortness of breath
- feeling weak
- fever
- cough
- cold-like symptoms (upper respiratory infection)
- nerve damage causing tingling, numbness or pain
- swollen hands, ankles or feet
- constipation
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of daratumumab. Call your doctor for medical advice about side effects. You can report any side effects to the FDA by calling 1-800-FDA-1088.
General information about the safe and effective use of daratumumab
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your healthcare provider or pharmacist for information about daratumumab that is written for healthcare professionals.
What are the active ingredients in daratumumab?
Active ingredient: daratumumab
Inactive ingredients: glacial acetic acid, mannitol, polysorbate 20, sodium acetate trihydrate, sodium chloride, and water for injection
POMALYST + dexamethasone + isatuximab-irfc
Information about POMALYST + dex + isatuximab-irfc does not appear in the POMALYST full Prescribing Information. Please talk to your doctor and/or see the isatuximab-irfc full Prescribing Information including Patient Information for a complete discussion of Important Safety Information at www.sarclisa.com.
Indication and Usage
Isatuximab-irfc is a prescription medicine used in combination with POMALYST and dexamethasone, to treat multiple myeloma in adults who have received at least two prior therapies including lenalidomide and a proteasome inhibitor. It is not known if isatuximab-irfc is safe and effective in children.
Do not receive isatuximab-irfc if you have a history of a severe allergic reaction to isatuximab-irfc or any of the ingredients in isatuximab-irfc.
See Patient Information in isatuximab-irfc full Prescribing Information for list of ingredients in isatuximab-irfc at www.sarclisa.com.
Possible side effects of isatuximab-irfc:
Isatuximab-irfc may cause serious side effects including:
- Infusion reactions. Infusion reactions are common with isatuximab-irfc and can sometimes be severe or life-threatening. Your healthcare provider will prescribe medicines before each infusion of isatuximab-irfc to help decrease your risk for infusion reactions or to help make any infusion reaction less severe. You will be monitored for infusion reactions during each dose of isatuximab-irfc. Your healthcare provider may slow down or stop your infusion, or completely stop treatment with isatuximab-irfc if you have an infusion reaction.
Get medical help right away if you develop any of the following symptoms of infusion reaction during or after an infusion of isatuximab-irfc: Shortness of breath, wheezing or trouble breathing, swelling of the face, mouth, throat, or tongue, throat tightness, palpitations, dizziness, lightheadedness, or fainting, headache, cough, rash or itching, nausea, runny or stuffy nose, chills. - Decreased white blood cell counts. Decreased white blood cell counts are common with isatuximab-irfc and certain white blood cells can be severely decreased. You may have an increased risk of getting certain infections, such as upper and lower respiratory tract infections and urinary tract infections. Your healthcare provider will check your blood cell counts during treatment with isatuximab-irfc. Your healthcare provider may prescribe an antibiotic or antiviral medicine to help prevent infection, or a medicine to help increase your white blood cell counts during treatment with isatuximab-irfc. Tell your healthcare provider right away if you develop any fever or symptoms of infection during treatment with isatuximab-irfc.
- Risk of new cancers. New cancers have happened in people during treatment with isatuximab-irfc. Your healthcare provider will monitor you for new cancers during treatment with isatuximab-irfc.
- Change in blood tests. Isatuximab-irfc can affect the results of blood tests to match your blood type. Your healthcare provider will do blood tests to match your blood type before you start treatment with isatuximab-irfc. Tell all of your healthcare providers that you are being treated with isatuximab-irfc before receiving blood transfusions.
The most common side effects of isatuximab-irfc in combination with POMALYST and dexamethasone include:
- lung infection (pneumonia)
- decreased red blood counts (anemia)
- upper respiratory tract infection
- decreased platelet counts (thrombocytopenia)
- diarrhea
These are not all the possible side effects of isatuximab-irfc. For more information, ask your healthcare provider or pharmacist.
Important Safety Information for isatuximab-irfc
Do not receive isatuximab-irfc:
- If you have a history of a severe allergic reaction to isatuximab-irfc or any of the ingredients in isatuximab-irfc.
See a full list of ingredients in isatuximab-irfc at www.sarclisa.com.
Before receiving isatuximab-irfc, tell your healthcare provider about all of your medical conditions, including if you:
- are pregnant or plan to become pregnant. Isatuximab-irfc may harm your unborn baby. You should not receive isatuximab-irfc during pregnancy.
- Females who are able to become pregnant should use an effective method of birth control during treatment and for 5 months after your last dose of
isatuximab-irfc. Talk to your healthcare provider about birth control methods that you can use during this time.
Tell your healthcare provider right away if you think you are pregnant or become pregnant during treatment with isatuximab-irfc. - are breastfeeding or plan to breastfeed. It is not known if isatuximab-irfc passes into your breast milk. You should not breastfeed during treatment with
isatuximab-irfc. Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Possible side effects of isatuximab-irfc:
Isatuximab-irfc may cause serious side effects including:
- Infusion reactions. Infusion reactions are common with isatuximab-irfc and can sometimes be severe or life-threatening.
Your healthcare provider will prescribe medicines before each infusion of SARCLISA to help decrease your risk for infusion reactions or to help make any infusion reaction less severe. You will be monitored for infusion reactions during each dose of isatuximab-irfc.
Your healthcare provider may slow down or stop your infusion, or completely stop treatment with isatuximab-irfc if you have an infusion reaction.
Get medical help right away if you develop any of the following symptoms of infusion reaction during or after an infusion of isatuximab-irfc: Shortness of breath, wheezing or trouble breathing, swelling of the face, mouth, throat, or tongue, throat tightness, palpitations, dizziness, lightheadedness, or fainting, headache, cough, rash or itching, nausea, runny or stuffy nose, chills. - Decreased white blood cell counts. Decreased white blood cell counts are common with isatuximab-irfc and certain white blood cells can be severely decreased. You may have an increased risk of getting certain infections, such as upper and lower respiratory tract infections and urinary tract infections.
Your healthcare provider will check your blood cell counts during treatment with isatuximab-irfc. Your healthcare provider may prescribe an antibiotic or antiviral medicine to help prevent infection, or a medicine to help increase your white blood cell counts during treatment with isatuximab-irfc. Tell your healthcare provider right away if you develop any fever or symptoms of infection during treatment with isatuximab-irfc. - Risk of new cancers. New cancers have happened in people during treatment with isatuximab-irfc. Your healthcare provider will monitor you for new cancers during treatment with isatuximab-irfc.
- Change in blood tests. Isatuximab-irfc can affect the results of blood tests to match your blood type. Your healthcare provider will do blood tests to match your blood type before you start treatment with isatuximab-irfc. Tell all of your healthcare providers that you are being treated with isatuximab-irfc before receiving blood transfusions.
The most common side effects of isatuximab-irfc in combination with POMALYST and dexamethasone include:
- lung infection (pneumonia)
- decreased red blood cell counts (anemia)
- upper respiratory tract infection
- decreased platelet counts (thrombocytopenia)
- diarrhea
These are not all the possible side effects of isatuximab-irfc. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects.

This website is best viewed
using the horizontal display
on your tablet device.

This website is best viewed
using the vertical display
on your mobile device.


