POMALYST® (pomalidomide) is an oral
medication that can be taken wherever is
convenient for you*
DOWNLOAD A TREATMENT CALENDAR
Keep track of your treatment schedule and doctor appointments using our Planning My Routine brochure.
DOWNLOAD NOWSample 28-day dosing calendar (POMALYST + dex).
Always follow your doctor’s instructions with regard to taking POMALYST with dex—even if those instructions are different from this calendar. The green areas of this calendar point out the days you should take POMALYST, while the orange areas point out the days for dexamethasone.
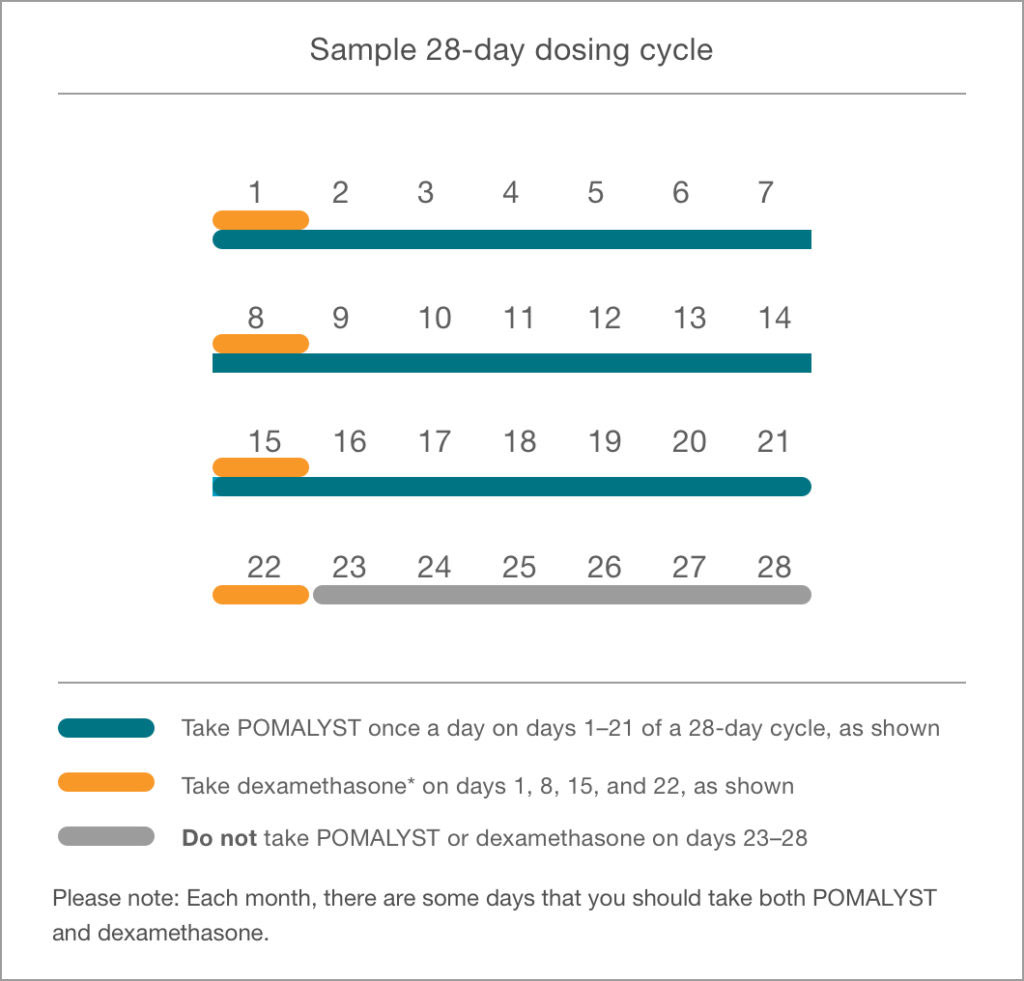
*This calendar is based on how POMALYST and dexamethasone were studied in the Phase 3 clinical trial.
How should I take POMALYST?
Important dosing information
- Take POMALYST exactly as prescribed, and follow all the instructions of the POMALYST REMS® program.
- Swallow POMALYST capsules whole with water 1 time a day. Do not break, chew, or open your capsules.
- POMALYST may be taken with or without food.
- Take POMALYST at about the same time each day.
- If you are on hemodialysis, take POMALYST after hemodialysis, on hemodialysis days.
- Do not open the POMALYST capsules or handle them any more than needed. If you touch a broken POMALYST capsule or the medicine in the capsule, wash the area of your body right away with soap and water.
- If you miss a dose of POMALYST and it has been less than 12 hours since your regular time, take it as soon as you remember. If it has been more than 12 hours, just skip your missed dose.
- Do not take 2 doses at the same time.
- If you take too much POMALYST, call your healthcare provider right away.
- Do not share POMALYST with other people. It may cause birth defects and other serious problems.
For more information about taking POMALYST, please see our FAQs page.
Additional important dosing information
Dosing modifications are sometimes necessary.
Your healthcare provider may tell you to decrease your dose, temporarily stop, or permanently stop taking POMALYST if you develop certain serious side effects during treatment with POMALYST.
For patients with severe renal impairment requiring dialysis:
Your doctor may recommend a lower dose (3 mg/day) or suggest that POMALYST be taken after your dialysis treatment on dialysis days.
Your doctor will tell you how to take POMALYST. Follow your doctor’s instructions carefully. Be sure to talk with your doctor or nurse if you have questions.

This website is best viewed
using the horizontal display
on your tablet device.

This website is best viewed
using the vertical display
on your mobile device.




