Based on results of a clinical trial, the FDA has approved the use of POMALYST with low-dose dexamethasone.
A clinical trial studied 455 patients whose multiple myeloma had stopped responding to at least 2 prior medicines, including REVLIMID and a PI. A total of 302 patients received POMALYST with low-dose dexamethasone, and 153 patients received high-dose dexamethasone alone.
The phase 3 clinical trial was designed to determine if POMALYST with low-dose dexamethasone could be an effective treatment for relapsed multiple myeloma.
MEDIAN OVERALL SURVIVAL (OS) RESULTS
Median Overall Survival: Think of median as the middle value of a set of data points. Overall survival measures the amount of time patients were alive following the start of the trial.
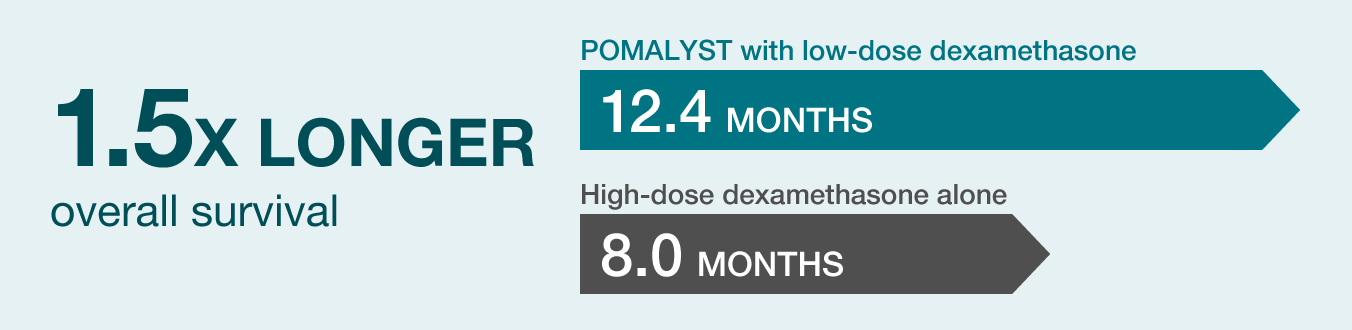

Study findings: Median OS for patients taking POMALYST with low-dose dexamethasone was 12.4 months. This is roughly 1.5 times longer than the 8 months that patients lived when they were given high-dose dexamethasone alone.
MEDIAN PROGRESSION-FREE SURVIVAL (PFS) RESULTS
Median Progression-Free Survival: Progression-free survival measures how long a patient lives without the disease getting worse. The main goal of the study was to measure median PFS.
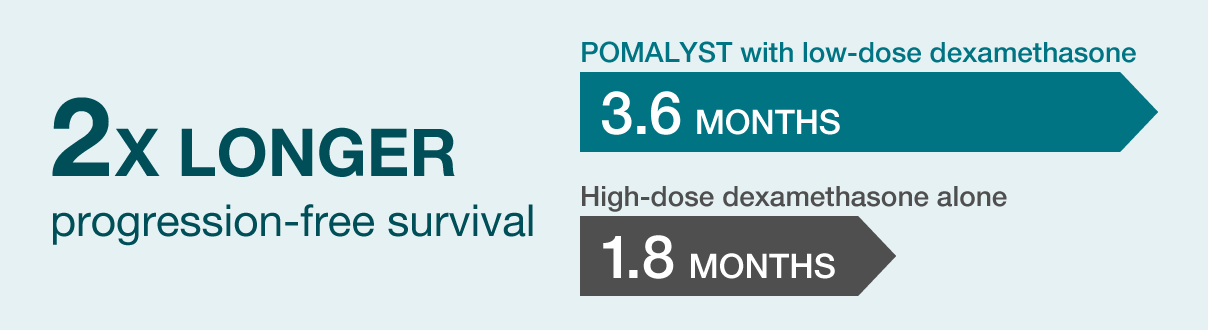

Study findings: Median PFS for patients taking POMALYST with low-dose dexamethasone was 3.6 months. This was 2 times longer than the 1.8 months that patients lived without their disease getting worse when they were given high-dose dexamethasone alone.
POMALYST may not work for everyone. Ask your doctor if POMALYST with dexamethasone is right for you. Individual results may vary.
Safety findings: Common side effects of POMALYST (≥30%) include low white blood cells, low red blood cells, tiredness and weakness, upper respiratory tract infection, low platelets, fever, shortness of breath, diarrhea, constipation, back pain, and nausea.
















