What to expect when multiple myeloma relapses

What to expect when multiple myeloma relapses

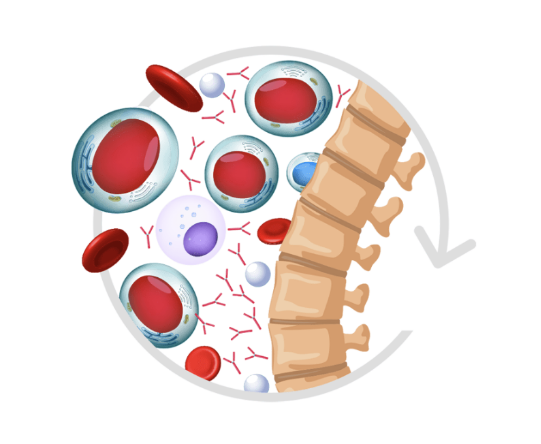



Join the support program for those who are taking POMALYST or those who want more information.
SIGN UP


This website is best viewed
using the horizontal display
on your tablet device.

This website is best viewed
using the vertical display
on your mobile device.